Dr Simon Cork, senior lecturer in physiology at Anglia Ruskin University, reviews the latest advances in drug treatment for obesity, including liraglutide, semaglutide and tirzepatide
Introduction
The quest to find effective and safe anti-obesity drugs has been long. Drugs have been introduced, only to be withdrawn after safety concerns, with many of these drugs targeting central appetite pathways. Phentermine and fenfluramine were introduced as combination drugs in the early 1990s but withdrawn in 1997 after it emerged that fenfluramine was found to cause pulmonary hypertension and altered cardiac valve morphology. Rimonabant and sibutramine followed, both being licenced in the mid-2000s and both targeting distinct neurotransmitter systems (cannabinoid and serotonin/noradrenaline respectively). However, both drugs were withdrawn from the market a few years later, after it was discovered that rimonabant increased the risk of suicidal ideation and sibutramine raised the risk of adverse cardiovascular events.
For the last decade, patients have been left with only one pharmacological option for tackling obesity: orlistat. While somewhat effective (with an average weight loss of around 8%), weight loss is compromised by adverse side effects, such as faecal incontinence and steatorrhea.
New era in drug treatment
In 2020, liraglutide (Saxenda) became the first anti-obesity drug to be approved for use by the NHS in over a decade, ushering in a new era in obesity treatment. Liraglutide is the first in a new class of anti-obesity drugs that mimic the effects of the endogenous hormone glucagon-like peptide-1 (GLP-1) to promote satiety and help facilitate weight loss. While effective for what is often an intractable condition, liraglutide had limited efficacy, leading to moderate weight loss of around 8% after 68 weeks1.
In March 2023, NICE recommended another GLP-1 analogue, semaglutide (Wegovy), for weight loss. The approval of semaglutide was met with much media coverage, in part because of celebrity endorsement. Clinical trials found semaglutide to have superior weight loss effects compared with liraglutide, with patients losing on average 16% body weight over 68 weeks2. More recently, tirzepatide (Mounjaro) has been approved for the treatment of obesity by the Medicines and Healthcare Regulatory Agency (MHRA) but is currently only available on the NHS for patients with Type 2 diabetes. Tirzepatide is a dual agonist, with actions against the GLP-1 receptor and glucagon receptor. Clinical trials demonstrate average weight loss of 20.9% on the highest doses after 72 weeks3.
Together, these drugs suggest we are entering a new era in obesity treatment. How do they work, and how should they be used?
Mechanism of action
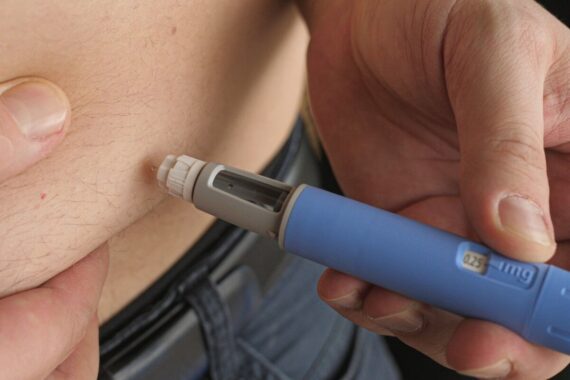
Liraglutide, semaglutide and tirzepatide are injectable agonists of the GLP-1 receptor. GLP-1 is an intestinal-derived hormone, which helps promote insulin release and suppress appetite. All three drugs are also licensed the treatment of type 2 diabetes (with Ozempic the licenced version of semaglutide for diabetes management).
It is the appetite suppressive actions of GLP-1 that make it such a potent target for the treatment of obesity. GLP-1 receptors are located in regions of the brain associated with both homeostatic feeding (the need to eat due to energy requirements) and hedonic feeding (eating due to the pleasure associated with certain foods). Because of this dual approach to suppressing hunger, GLP-1 analogues have a powerful effect in suppressing the increased appetite that is associated with weight loss.
Tirzepatide is also an agonist of the glucagon receptor. Like GLP-1, glucagon has an appetite suppressive effect, acting via the vagus nerve. Pharmacological administration of glucagon has also demonstrated increased energy expenditure4, potentially contributing to the superior weight loss associated with tirzepatide compared with GLP-1 agonists alone.
Our bodies’ physiological response to weight loss is to promote weight gain (through increased hunger, decreased motivation to move and decreased metabolic activity), thus maintaining body weight. Because hunger is such a powerful motivator of behaviour, it often causes failure when weight loss is attempted through behavioural modification alone.
The drugs do not in themselves cause weight loss. Patients still need to change their diet and increase exercise to see any meaningful change in body weight.
What are the drawbacks?
Because of the mechanism of action of these drugs, the brake they apply to the hunger associated with weight loss lasts only as long as the patient is taking the drug. A study which followed patients after semaglutide withdrawal found all patients regained at least two-thirds of the weight they had previously lost within a year5. The same study also demonstrated that the decrease in blood pressure, cholesterol and HbA1c levels associated with semaglutide reverted to baseline following drug withdrawal. We don’t know if these parameters would have reverted to baseline if patients had been able to maintain their weight loss. Similar findings have also been demonstrated for tirzepatide6.
This is particularly important because NICE guidelines for the prescribing of liraglutide and semaglutide place a two-year limit on its use. This two year limit is in place in part due to its requirement to be prescribed within a Tier 3 specialist weight management service, for which patients are limited to 2 years access. There is also evidence that treatment after 2 years would increase the incremental cost-effectiveness ratio (ICER), meaning long term treatment may not be cost effective. Furthermore, clinical trials on the safety and efficacy of these drugs have not exceeded 2 years, therefore long-term safety profiles are not available. These measures may change in the future, with plans to widen prescribing rights of anti-obesity medications to GPs, meaning the requirement for referral to Tier 3 services is no longer required. Whether tirzepatide will face similar time limiting restrictions has not yet been decided.
Currently, semaglutide and tirzepatide are only available as once weekly injections, although oral semaglutide is showing promise in late stage trials7. Side effects are generally mild and transient (nausea, vomiting, diarrhoea and constipation being the most common) but these can mean people stop taking them. More serious side effects have been rarely reported, including cholelithiasis, although this is quite commonly associated with rapid weight loss by any means.

Perhaps the most acute issue facing liraglutide and semaglutide is access. A supply notification issued by the Department of Health and Social Care (DHSC) in March 2024 prevents new patients from being prescribed either of these drugs for the treatment of type 2 diabetes or obesity. It is anticipated that supply issues will last the remainder of 2024. These supply issues are in large part because GLP-1 receptor agonists have become a victim of their own success, with off label prescribing for the treatment of obesity largely to blame. The consequence of this is that patients who have been taking these drugs for many years to treat their diabetes are struggling to access reliable supplies.
Where do these drugs fit into practice?
So, who are these new generation of drugs aimed at? Therapies which are targeted at patients with overweight or lower levels of obesity (obesity class I) are lacking in efficacy.
Lifestyle interventions are the first line treatment for anyone seeking medical help with weight, however for the majority of these patients, diet and exercise are seldom effective.
Orlistat continues to be the first line pharmacotherapy for many, given its low BMI starting criteria (30kg/m2 in patients without comorbidities), but the relatively modest weight loss and unsociable side effects limit its appeal in many patients.
Bariatric surgery remains the most effective method of inducing long-term weight loss, however the threshold for referral to bariatric surgery is very high (BMI >40kg/m2 in patients without comorbidities). Likewise, surgery is not without complications or side effects and is therefore not the preferred choice for many.
Effective GLP-1 based pharmacotherapies bridge a gap between those who could be treated with current treatments and those for whom bariatric surgery is neither desirable nor clinically possible. As novel and even more effective drugs make their way down the clinical trial pipelines (see below), the future need for bariatric surgery for many patients will be unnecessary.
What does the future hold?
The history of anti-obesity pharmacotherapy is littered with relatively effective, yet ultimately unsafe drugs. GLP-1 agonists are both effective and largely safe. It is therefore unsurprising that many pharmaceutical companies are racing to produce the next drug in what is a lucrative market.
Oral GLP-1 agonists are showing promise in Phase 3 clinical trials, with Novo Nordisk reporting oral semaglutide results in a mean weight loss of 15.1% over 68 weeks7, and Eli Lilly reporting their own oral GLP-1 receptor agonist, Orforglipron, resulting in 14.7% weight loss over 36 weeks8. Pfizer have also joined the race, with Phase 2b unpublished trial data demonstrating an 8-13% weight loss over 32 weeks with their own oral GLP-1 receptor agonist.
Retatrutide, a triple action agonist developed by Eli Lilly, acts on the GLP-1, glucagon and glucose-dependent insulinotropic polypeptide (GIP) receptors. Phase 2 trials have recently concluded, demonstrating an impressive 24.2% weight loss after 48 weeks, with concomitant improvements in blood pressure, HbA1c and triglycerides9.
Amycretin, a co-agonist of the GLP-1 and amylin receptors has demonstrated 17.1% weight loss in an as-yet unpublished Phase 1 trial according to a press release from Novo Nordisk. If later trials show promise, amycretin would also have the advantage of being an oral medication, which is likely to improve adherence from patients.
Finally turning a page
The development of multiple novel, effective and safe anti-obesity therapies will, in time, provide patients with increased choice and alleviate well documented shortages in GLP-1 based therapies.
The narrative around obesity may also come to change. Obesity is a lifelong condition, which requires lifelong management. Hitherto management has relied almost exclusively on self-motivation, with patients often fighting a losing biological battle. Guidelines will have to change to recognise this. Whether that requires patients to stay on these drugs for life (in the same way that one would expect a hypertensive patient to remain on anti-hypertensives), or whether a step-down approach is needed, must be addressed. Currently patients face a ‘cliff edge’ of treatment cessation after two years.
Dr Simon Cork is a senior lecturer in physiology at Anglia Ruskin University
References
- Pi-Sunyer, X., Astrup, A., Fujioka, K., et al. A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. New England Journal of Medicine 2015,373(1),11–22
- Wilding, J.P.H., Batterham, R.L., Calanna, S., et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. New England Journal of Medicine 2021,384(11),989–1002
- Jastreboff, A.M., Aronne, L.J., Ahmad, N.N et al. Tirzepatide Once Weekly for the Treatment of Obesity. New England Journal of Medicine 2022,387(3),205–216
- Nair, K.S. Hyperglucagonemia Increases Resting Metabolic Rate In Man During Insulin Deficiency*. The Journal of Clinical Endocrinology & Metabolism 1987,64(5), pp.896–901.
- Wilding, J.P.H., Batterham, R.L., Davies, M., et al. Weight regain and cardiometabolic effects after withdrawal of semaglutide: The STEP 1 trial extension. Diabetes, Obesity and Metabolism 2022,24(8),1553–1564
- Aronne, L.J., Sattar, N., Horn, D.B., et al. Continued Treatment With Tirzepatide for Maintenance of Weight Reduction in Adults With Obesity. JAMA 2024,331(1), p.38
- Knop, F.K., Aroda, V.R., do Vale, R.D et al. Oral semaglutide 50 mg taken once per day in adults with overweight or obesity (OASIS 1): a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet 2023,402(10403),705–719
- Wharton, S., Blevins, T., Connery, L., et al. Daily Oral GLP-1 Receptor Agonist Orforglipron for Adults with Obesity. New England Journal of Medicine, 2023, 389(10), pp.877–888.
- Jastreboff, A.M., Kaplan, L.M., Frías, J.P., et al. Triple–Hormone-Receptor Agonist Retatrutide for Obesity — A Phase 2 Trial. New England Journal of Medicine 2023,389(6),514–526.

















Said it before, but I’ll say it again———-”ban all cookery programs and T.V. chefs”